|
Last updates: |
|
The EU actively promotes the use of information and telecommunication technologies for digital health and care that provide tools and services to improve the prevention, diagnosis, treatment, monitoring and management of health-related problems and to monitor and managing lifestyle habits that impact health.
Digital health and care improve the quality of health care and the efficiency of the health sector.
The transition to the Digital Age: Health
"A Europe fit for the digital age" is one of the Commission's six policy priorities for 2019-2024. Building on previous initiatives that improve the creation of a digital single market, the digital transition should be something that benefits everyone, putting people first and opening new opportunities for businesses. Health is one of the sectors included in this agenda, given the potential benefits that digital services have to offer to citizens and businesses in this area.
The transition to a digital age in healthcare is a process that began a long time ago.
As always happens, sometimes there are unexpected events that accelerate the transformation of society. This is the case with healthcare. Shaken by the pandemic of the new Coronavirus that led to the disease called COVID-19, she had to put together all her skills to find the most suitable solutions. In reality, it has discovered that it still has very few technological resources and in fact the Coronavirus pandemic has claimed about 1 million 219 thousand deaths in Europe alone in 20 months and has frozen the economy and social activities.
In fact, social distancing was an obligatory consequence of the pandemic, but also the only possible way to stem infections and deaths. Diagnosis, treatment and treatment are almost the same as they were 50 years ago.
The disaster of recent months has been treasured and it has been understood how much an e-health could help, where patient and doctor are in constant contact even from a distance and where there are no obstacles to bring treatment to a patient or to make a diagnosis. Quickly.
2018: EU publishes the Strategy on the Digital Health and Care in Europe
|
In 2018, the European Commission published a Communication on the Digital transformation of healthcare. eHealth, telemedicine and other digital technologies such as 4G/5G mobile communications, artificial intelligence and supercomputing offer new opportunities to transform healthcare systems. Pillar 1: Secure data access and sharingTo facilitate greater acces to cross-border healthcare, the Commission is building the eHealth Digital Service Infrastructure to allow e-prescriptions and patient summaries to be exchangedSearch for available translations of the preceding linkEN••• between healthcare providers. The first cross-border exchanges started in 2019, with the goal of having all the other EU countries on board by 2025. In the longer term, the Commission is working towards establishing a European electronic health record exchange format that is accessible to all EU citizens. Pillar 2: Connecting and sharing health data for research, faster diagnosis and improved healthThe second pillar of the 2018 Communication intends to tap into the huge potential of health data to support medical research with the aim of improving prevention, diagnosis, treatments, drugs and medical devices. Pillar 3: Strengthening citizen empowerment and individual care through digital servicesDigital services can empower citizens, making it easier for them to take a greater role in the management of their own health from following prevention guidelines and being motivated to lead healthier lifestyles, to managing chronic conditions and providing feedback to healthcare providers. Health systems will also benefit from innovative care models that use telehealth and mHealth to address the rising demand for healthcare, helping to shift progessively towards integrated and personalised care systems. |
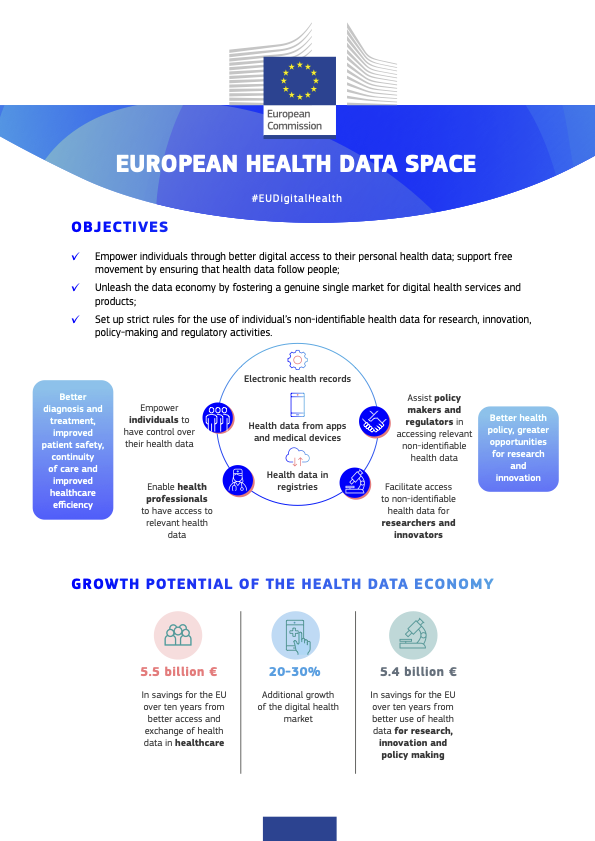
2022 Launch of the European Health Data Space (EHDS) for people and science
|
On 3 May 2022, the European Commission has opened a new phase for the digitisation of personal health data, with the launch of a Proposal for a Regulation on the European Health Data Space (EHDS).
The EHDS will help EU countries to implement an advanced health service that all people can benefit from. In fact, the process has already begun for some time. In many EU countries, digital medical records are already functioning for many citizens, of which medical prescriptions are only one aspect and which will be increasingly integrated with the medical profile of each citizen. When fully operational, the EHDS will allow medical staff and citizens to check and use their health data in their home country or in other Member States. This, in addition to improving the quality of health care, will promote the internal market for digital health services and products. It will also be of help to the entire research & innovation, providing coherent and up-to-date data, as well as support for the development of policies and regulatory activities. EHDS aims primarily to:
The Proposal for a Regulation is dealing with:
The Proposal for the Regulation on the European Health Data Space (EHDS) has been negotiated by Council of the EU and European Parliament in March 2024. Here the text. The consensus achieved outlines clear regulations for the utilization of health data to improve healthcare delivery, research, innovation, and policy-making. These rules are designed to exploit the benefits of safe and secure health data exchange, usage, and reuse, all while adhering to the EU's stringent data protection standards. Key provisions under the new regulations include:
The European Parliament and the Council are set to formally adopt the new Regulation, which will be applied in various stages, differentiated by use case and data type. |
Area 1 - Citizens' secure data access and sharing
Interoperability of Member states' electronic health record systems by supporting the development and adoption of a European electronic health record exchange format.
Actions in progress or completed:
- The Commission Implementing Decision 2019/1765 of 22 October 2019 providing the Rules for the establishment, the management and the functioning of the network of national authorities responsible for eHealth. It is about clarifying the role of the eHealth network in the governance of the eHealth digital service infrastructure and its operational requirements, as well as improving patient data interoperability and citizen access.
- The Commission recommendation (EU) 2019/243 of 6 February 2019 on a European Electronic Health Record exchange format while monitoring implementation of relevant EU legislation and considering other measures in the future if needed. Such specifications should also address citizens' access to electronic health records and aspects related to the implementation of appropriate data protection safeguards and security of patient health data in compliance with the General Data Protection Regulation.
- Further support the eHealth Digital Service Infrastructure to enable new services for people, such as exchange of electronic health records using the specifications of the European electronic health record exchange format, and the use of the data for public health and research. Preparatory work was conducted already in 2020, through a series of workshops and a study + annex (see below) to provide a framework for the primary (prescription and dispensing of medicines) and secondary (research & diagnosis ) use of health data in the Member States. This particularly through: a mapping of GDPR implementation in the health sector in the different countries, including an overview of the legal and technical modalities applicable to health data sharing for primary and for secondary uses in the EU countries; an overview of the existing governance structures for secondary use of health data in the EU countries; recommendations for possible actions, legislative and non-legislative, at EU level to facilitate health data sharing across the EU for primary and for secondary uses.
- Mobilise funds of the multi-annual financial framework, to encourage further collaboration between Member States and between regions on the cross-border exchange of health data and its possible expansion (notably to full electronic health records and other new services).
Area 2 - Connecting and sharing health data for research, faster diagnosis and improved health
The creation of a European Data Space is one of the priorities of the Commission 2019-2025, including the health sector. A common European Health Data Space will allow to exchange and access to different types of health data (electronic health records, genomics data, data from patient registries etc.). So, not only to support primary use of data (healthcare delivery) but also the secondary use of data (health research and health policy making purposes).
The Commission, in collaboration with the Member States, is engaged in the preparatory work and development of the European Health Data Space. Member States will be supported by a new “Joint Action for the European Health Data Space” (website) set up to help the Members States and the Commission facilitate the sharing of health data for public health, treatment, research and innovation in Europe.
The European Health Data Space will be built on 3 main pillars:
- a strong system of data governance and rules for data exchange
- data quality
- strong infrastructure and interoperability
Actions in progress or completed:
- Set up a mechanism for the voluntary coordination of authorities and other stakeholders to share data and infrastructure for prevention and personalised medicine research. This includes a European network on genomics, and seeking to link also with ongoing '-omics' and human cell mapping initiatives. European Reference Networks (ERNs) are virtual networks involving healthcare providers across Europe. European Reference Networks (ERNs) were created as virtual networks involving healthcare providers across Europe. Today, 24 ERNs are involving 25 European countries included Norway, over 300 hospitals with over 900 healthcare units and covering all major disease groups.
- Support the development of technical specifications for secure access and cross-border exchange of genomic and other health datasets within the internal market for research purposes. This is to facilitate interoperability of relevant registries and databases in support of personalised medicine research.
- Launch pilot actions, pooling data and resources across the EU, to demonstrate the benefits of advancing research, disease prevention, personalised medicine, health technology assessment, as well as clinical and regulatory decision making.
- Mobilise funds of the multi-annual financial framework
See some examples of eHealth Network guideline issued by the voluntary EU network:
Area 3 - Strengthening citizen empowerment and individual care through digital services
Actions in progress or completed:
- Support cooperation to stimulate the supply and uptake of digital health by promoting common principles for validating and certifying health technology.
- Support the exchange of innovative and best practices, capacity building and technical assistance for health and care authorities (for using open standards and interoperable digital solutions to promote health, prevent and manage chronic conditions, empower people and centre care on the person), with financial support from Horizon Programmes, the Structural Reform Support Programme and the third EU Health programmes.
- Raise awareness about innovative procurement and investment possibilities for digital transformation in public health and healthcare, mobilising relevant EU programmes and financial instruments, collaborating with the European Investment Bank and investor networks, and considering further support, including possible co-investment approaches, under the next multi-annual financial framework.
- Promote knowledge and skills of citizens, patients and health and care professionals in using digital solutions in collaboration with health professional organisations and academia.
Other benefits for patients
|
As described before, when national health systems will be interconnected and will use networks to exchange data, the benefits for patients are immense, as seen before. In addition, the interconnection also facilitates the ability to contact a hospital other than your own country, because you are traveling. The Directive 2011/24/EU on patients' rights in cross-border healthcare sets out the rights for patients and the rules for exercising them. The Directive is dealing with:
The Directive:
Check the 2019 report on the application of the Directive ------------------------> Cross-border Healthcare Expert Group The Cross-border Healthcare Expert Group brings together healthcare representatives from all EU countries to assist the European Commission with the implementation of the Directive. It provides the Commission with advice and expertise, and national authorities with a forum to exchange their experiences of the Directive. Are you seeking healthcare in another EU Member State? Before, read your rights and what it is important to know:
Related information |
Source: European Union, http://www.europa.eu/, 1998-2024
|
Brussels - Milano - Nice - Tokyo
|
eEuropa Belgium
Avenue Louise, 367 1050 Brussels BELGIUM Bld. Franck Pilatte, 19 bis
06300 Nice FRANCE YONO HOUSE 9-1 KAMIOCHIAI, SAITAMA-SHI, SAITAMA-KEN 〒 338-0001 JAPAN Via S. Veniero 6 20148 Milano ITALY |
All rights reserved - © Copyright eEuropa Belgium 2020-2024