Medical devices & IVDs: EU Legislation
In the field of medical devices and in Vitro Diagnostic Medical devices (IVD) the EU is highly competitive and innovative, with many small and medium-sized enterprises. The EU has put in place a regulatory framework both to protect patients' health and to ensure the smooth functioning of the internal market.
European legislation
|
About 500,000 medical devices and IVDs can be counted on the EU market. For example, patches, contact lenses, X-ray machines, pacemakers, breast implants, software apps, and hip implants. IVDs are used to perform sample testing, and examples include blood tests for HIV, pregnancy tests, and blood glucose monitoring systems for diabetics. Legislation Medical devices and in vitro diagnostic medical devices are currently regulated by 1 Regulation and (for short time) 3 Directives:
The 3 Directives are progressively replaced 2 Regulations:
|
At the end of the transitional periods:
Legislative Act |
Amending |
Repealing |
Fully applicable |
Implementing Decisions |
Regulation (EU) 2017/745 on medical devices (MDR), |
|
Council Directives:
|
26 May 2021 |
|
// |
|
26 May 2022 |
The European Commission informs that:
The new regulations contain a series of extremely important improvements to modernise the current system. Among them are:
All actors involved with medical devices, from their manufacture to their use, will have to comply with the new regulations by May 2021 (May 2022 for in vitro diagnostic medical devices). It is important that all actors are fully aware of the changes and start preparing for the implementation of the new regulations as soon as possible.
Be aware that the European Commission is not responsible for content provided by non-Commission websites.
The new regulations contain a series of extremely important improvements to modernise the current system. Among them are:
- stricter previous control for high-risk devices via a new pre-market scrutiny mechanism with the involvement of a pool of experts at EU level
- reinforcement of the criteria for designation and processes for oversight of notified bodies
- inclusion of certain aesthetic devices that present the same characteristics and risk profile as analogous medical devices under the scope of the regulations
- a new risk classification system for in vitro diagnostic medical devices in line with international guidance
- improved transparency through a comprehensive EU database on medical devices and a device traceability system based on a unique device identification
- introduction of an ‘implant card’ for patients containing information about implanted medical devices
- reinforcement of the rules on clinical evidence, including an EU-wide coordinated procedure for authorising multi-centre clinical investigations
- strengthening of post-market surveillance requirements for manufacturers
- improved coordination mechanisms between EU countries in the fields of vigilance and market surveillance
All actors involved with medical devices, from their manufacture to their use, will have to comply with the new regulations by May 2021 (May 2022 for in vitro diagnostic medical devices). It is important that all actors are fully aware of the changes and start preparing for the implementation of the new regulations as soon as possible.
Be aware that the European Commission is not responsible for content provided by non-Commission websites.
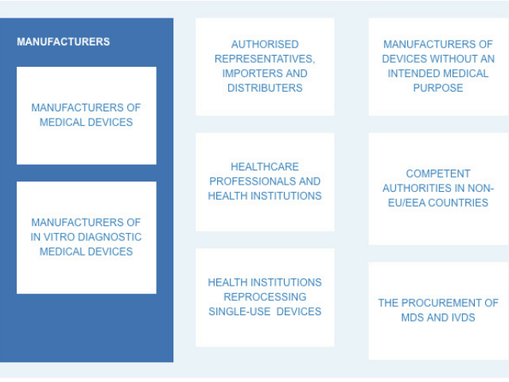
Summarised view of the main areas of activities in the medical Devis sector
Check the rules in you areas of activity (provided by the Commission):
Harmonised European standards
Harmonised European standards under the medical devices Regulations are developed by CEN and Cenelec, the two European standardisation organisations (Regulation (EU) 1025/2012). Once their references are published by the Commission in the Official Journal of the European Union, the voluntary use of those standards confer presumption of conformity with the requirements of the Regulations they aim to cover.
The first publications of references of harmonised European standards under the medical devices regulations are available:
- Commission Implementing Decision (EU) 2021/1182 of 16 July 2021 on the harmonised standards for medical devices drafted in support of Regulation (EU) 2017/745
- Commission Implementing Decision (EU) 2021/1195 of 19 July 2021 on the harmonised standards for in vitro diagnostic medical devices drafted in support of Regulation (EU) 2017/746
The lists of references of harmonised European standards under the medical devices Regulations published in the OJ are available on the standardisation webpages on healthcare engineering: Medical devices, In vitro diagnostic medical devices.