Restrictions are an instrument to protect human health and the environment from unacceptable risks posed by chemicals. Restrictions are normally used to limit or ban the manufacture, placing on the market (including imports) or use of a substance, but can impose any relevant condition, such as requiring technical measures or specific labels.
A restriction may apply to any substance on its own, in a mixture or in an article, including those that do not require registration, for example, substances manufactured or imported below one tonne per year or certain polymers.
On-site isolated intermediates, substances used in scientific research and development, and substances only posing risks to human health from their use in cosmetics are exempted from those substances to which REACH restriction applies.
A restriction may apply to any substance on its own, in a mixture or in an article, including those that do not require registration, for example, substances manufactured or imported below one tonne per year or certain polymers.
On-site isolated intermediates, substances used in scientific research and development, and substances only posing risks to human health from their use in cosmetics are exempted from those substances to which REACH restriction applies.
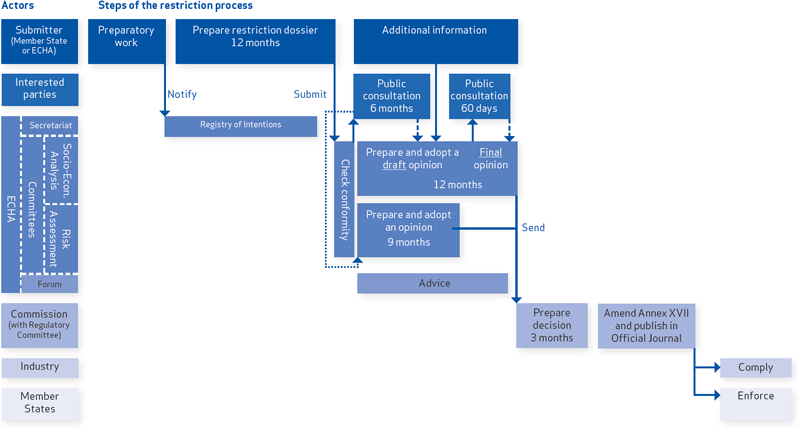
Restriction Process
Restriction procedure
A Member State, or ECHA, at the request of the European Commission, can start the restriction procedure when they are concerned that a certain substance poses an unacceptable risk to human health or the environment. ECHA can also propose a restriction on articles containing substances that are on the Authorisation List (Annex XIV).
The intention to prepare a restriction proposal is made public in the registry of intentions before the proposal file itself is prepared so as to give an advance warning.
The dossier proposing the restriction contains background information such as the identity of the substance and justifications for the proposed restrictions. It includes the identified risks, any information on alternatives to the substance and the costs, as well as the environmental and human health benefits, resulting from the restriction.
The dossier needs to be prepared according to the REACH Regulation (Annex XV) and has to be submitted to ECHA within 12 months of the intention to prepare the proposal was notified.
Committees' opinions
Upon receiving the dossier ECHA's committees check whether the proposal conforms to the requirements of Annex XV. If it does, the dossier will be made publicly available for consultation (excluding any commercially confidential information). Interested parties can then comment on the restriction within six months of its publication on the ECHA website.
Within nine months of that same publication date, ECHA's Committee for Risk Assessment (RAC) will give its opinion on whether the suggested restriction is appropriate in reducing the risk to human health or the environment based on the dossier and the comments received during the public consultation.
At the same time, the Committee for Socio-economic Analysis (SEAC) prepares an opinion about the socio-economic impacts of the suggested restrictions, taking into account the comments and socio-economic analyses submitted by interested parties. All comments on the SEAC draft opinion should be submitted within 60 days of its publication. SEAC will then adopt its final opinion, taking the comments into account, within 12 months of the start of the first public consultation on the restriction proposal.
Besides the two groups above the Forum of enforcement authorities from the Member States may provide advice to the committees on the enforceability of the proposed restriction.
Decision
The two opinions of ECHA's committees contribute to the decision of the European Commission, which will then take a balanced view of the identified risks and of the benefits and costs of the proposed restriction.
Within three months of receiving two committees' opinions, the Commission will provide a draft amendment to the list of restrictions in Annex XVII to REACH. The final decision is taken in a comitology procedure with scrutiny involving the Member States and the European Parliament.
Enforcement
Once the restriction has been adopted, industry must comply. That means all including manufacturers, importers, distributors, downstream users and retailers.
The Member States are responsible for enforcing the restriction.
A Member State, or ECHA, at the request of the European Commission, can start the restriction procedure when they are concerned that a certain substance poses an unacceptable risk to human health or the environment. ECHA can also propose a restriction on articles containing substances that are on the Authorisation List (Annex XIV).
The intention to prepare a restriction proposal is made public in the registry of intentions before the proposal file itself is prepared so as to give an advance warning.
The dossier proposing the restriction contains background information such as the identity of the substance and justifications for the proposed restrictions. It includes the identified risks, any information on alternatives to the substance and the costs, as well as the environmental and human health benefits, resulting from the restriction.
The dossier needs to be prepared according to the REACH Regulation (Annex XV) and has to be submitted to ECHA within 12 months of the intention to prepare the proposal was notified.
Committees' opinions
Upon receiving the dossier ECHA's committees check whether the proposal conforms to the requirements of Annex XV. If it does, the dossier will be made publicly available for consultation (excluding any commercially confidential information). Interested parties can then comment on the restriction within six months of its publication on the ECHA website.
Within nine months of that same publication date, ECHA's Committee for Risk Assessment (RAC) will give its opinion on whether the suggested restriction is appropriate in reducing the risk to human health or the environment based on the dossier and the comments received during the public consultation.
At the same time, the Committee for Socio-economic Analysis (SEAC) prepares an opinion about the socio-economic impacts of the suggested restrictions, taking into account the comments and socio-economic analyses submitted by interested parties. All comments on the SEAC draft opinion should be submitted within 60 days of its publication. SEAC will then adopt its final opinion, taking the comments into account, within 12 months of the start of the first public consultation on the restriction proposal.
Besides the two groups above the Forum of enforcement authorities from the Member States may provide advice to the committees on the enforceability of the proposed restriction.
Decision
The two opinions of ECHA's committees contribute to the decision of the European Commission, which will then take a balanced view of the identified risks and of the benefits and costs of the proposed restriction.
Within three months of receiving two committees' opinions, the Commission will provide a draft amendment to the list of restrictions in Annex XVII to REACH. The final decision is taken in a comitology procedure with scrutiny involving the Member States and the European Parliament.
Enforcement
Once the restriction has been adopted, industry must comply. That means all including manufacturers, importers, distributors, downstream users and retailers.
The Member States are responsible for enforcing the restriction.
Restriction phases - The EC Guide
- I Phase - Preparation and submission of a restriction proposal
- II-A Phase - Consultations
- II-B Phase - Opinion development
- III Phase - Decision and follow-up
Source: European Union, http://www.europa.eu/, 1998-2024
|
Brussels - Milano - Nice - Tokyo
|
eEuropa Belgium
Avenue Louise, 367 1050 Brussels BELGIUM Bld. Franck Pilatte, 19 bis
06300 Nice FRANCE YONO HOUSE 9-1 KAMIOCHIAI, SAITAMA-SHI, SAITAMA-KEN 〒 338-0001 JAPAN Via S. Veniero 6 20148 Milano ITALY |
All rights reserved - © Copyright eEuropa Belgium 2020-2024